News Story
UMD Chemist Pioneers Tool for Eavesdropping on Neurons

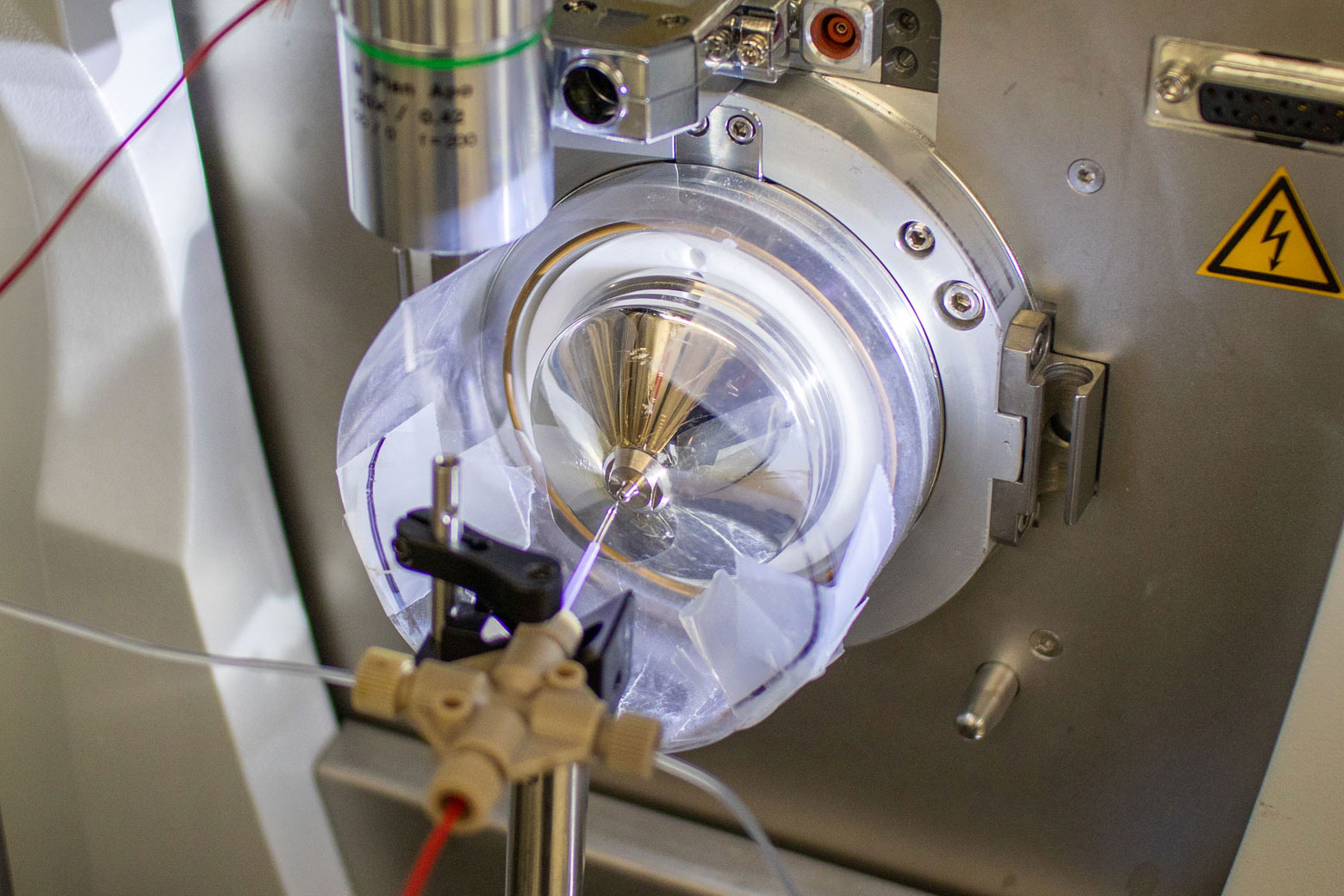
The ultrasensitive high-resolution mass spectrometer in the Nemes lab. Initial funding to build the instrument was provided by the Arnold and Mabel Beckman Foundation. Photo by Nathaniel Underland.
Researchers at the University of Maryland have developed a technological breakthrough in analytical neuroscience: the ability to profile the proteins inside of a single live neuron. The scientific advancement will improve understanding of the brain during normal development and advance diagnostics and therapeutics for diseases such as Alzheimer’s and Parkinson’s.
The technical milestone was achieved with a custom-built ultrasensitive high-resolution mass spectrometer. Built in-house, the instrument features a sensitivity sufficient to detect hundreds of different proteins in individually characterized neurons in a live brain section. The breakthrough was featured on the January 25, 2022 cover of Analytical Chemistry.
“This paper expands the analytical toolbox of neuroscience,” said Peter Nemes, the corresponding author of the study and Associate Professor in the Department of Chemistry and Biochemistry. “It lays the groundwork for a proteomic-driven classification of cell types in the brain.”
Although scientists have possessed tools to detect proteins in cells for years, they have lacked the technology to identify and quantify diverse proteins in tissue-embedded, characterized cells. The advanced ultrasensitive high-resolution mass spectrometry instrument developed by the Nemes lab introduces a newfound capacity to detect the diverse complement of molecules in individual cells—in this case, in neurons whose electrical activity has been functionally characterized. Because the function of any cell, including a neuron, intimately depends upon the proteins it generates, the novel ability of the instrument to identify and quantify hundreds of different proteins is vital to understanding the development and function of brain cells.
In bioanalytical chemistry, mass spectrometry provides information about the molecular composition of a sample—what is present and in what quantity—by “weighing” its molecules. The challenge for neuroscience has been to extract and detect the proteins generated by a cell from a very small sample.
To meet this challenge, Nemes refined the sensitivity of his mass spectrometer down to the scale of a single neuron—about 15-50 micrometers, or less than the width of an average human hair.
“There is enormous potential for patch-clamp proteomics,” said Elizabeth Quinlan, Professor in the Department of Biology, the Clark Leadership Chair in Neuroscience, and Director of the Brain and Behavior Institute (BBI). “There are so many exciting applications for Peter’s method to identify the full complement of proteins in physiologically characterized neurons, including for cells grown in culture, in brain slices, and even in the whole brain. I encourage interested UMD investigators to reach out to Peter to discuss new applications.”
Nemes’ technological breakthrough laid the foundation for a 2019 BBI seed grant with Najib El-Sayed, Professor in the Department of Cell Biology and Molecular Genetics with a joint appointment in the University of Maryland Institute for Advanced Computer Studies, and Colenso Speer, Assistant Professor in the Department of Biology, as well as for a 2021 MPower seed grant with El-Sayed, Speer, and Joshua Singer, Professor and Interim Chair of the Department of Biology. In both projects, Nemes used the novel instrument to identify differences in the protein makeup of the suprachiasmatic nucleus, an area of the brain that regulates circadian rhythm and whose disruption has been implicated in neurodegenerative diseases. What the teams obtained was a novel dataset. They identified an array of proteins that were dysregulated compared to normal functioning and that neuroscientists had not previously believed to contribute to neurodegeneration.
Now, Nemes’ development of ultrasensitive high-resolution mass spectrometry for individual neurons is poised to elucidate the molecular basis for functional differences between neurons.
A custom-built ion source on the mass spectrometer charges the biomolecules so that the Nemes lab can identify and quantify their mass and abundance. Photo by Nathaniel Underland.
“Once we can characterize the proteome of a neuron, we can think outside of the proverbial box,” said Nemes. “The scalability of high-resolution mass spectrometry across animal models opens the door to asking many basic neuroscience questions, particularly questions with translational implications for human health. How many other important proteins have we overlooked due to a lack of technological capability? Are we missing any key culprits of neurodegenerative diseases, such as other dysregulated proteins, simply because they do not fit into the canonical understanding of the brain?”
Nemes envisions the continued development of cutting-edge technologies and is keen to identify broad applications of patch-clamp proteomics through further collaborations at UMD.
“What’s exciting for us in College Park are the numerous colleagues working in different facets of neuroscience and developmental neurobiology who think strategically about integrating complementary expertise to pursue big problems in neuroscience with grand technologies,” said Nemes. “These teams can blossom really quickly, and they can have a major impact as they tackle pressing challenges in science and society with fresh—and even transformative—insights.”
###
Sam B. Choi, a recent Ph.D. graduate in Chemistry, and Abigail Polter of the George Washington University School of Medicine and Health Sciences, also co-authored the study.
The work was supported by the Arnold and Mabel Beckman Foundation Beckman Young Investigator Grant.
The research paper, “Patch-Clamp Proteomics of Single Neurons in Tissue Using Electrophysiology and Subcellular Capillary Electrophoresis Mass Spectrometry,” Sam B. Choi, Abigail M. Polter, and Peter Nemes, was published on January 25, 2022 in Analytical Chemistry.
Writer: Nathaniel Underland, underlan@umd.edu
About the Brain and Behavior Institute: The mission of the BBI is to maximize existing strengths in neuroscience research, education and training at the University of Maryland and to elevate campus neuroscience through innovative, multidisciplinary approaches that expand our research portfolio, develop novel tools and approaches and advance the translation of basic science. A centralized community of neuroscientists, engineers, computer scientists, mathematicians, physical scientists, cognitive scientists and humanities scholars, the BBI looks to solve some of the most pressing problems related to nervous system function and disease.
Published February 14, 2022